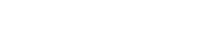How COVID-19 Vaccination Hesitancy Affects Your Benefits and What to Do About It
-
 Joshua Halperin, PharmD, Senior Clinical Director
Joshua Halperin, PharmD, Senior Clinical Director
- 10 min read

The U.S. has made huge strides in combating the COVID-19 pandemic by continuing to follow safety protocols that minimize the spread of the virus. In addition, the widespread availability of vaccinations for those who are 6 months and older has helped slow infection rates and the severity of illness. As a result, daily death rates, hospitalizations and new infections are all in decline. The medical community is in agreement that vaccination is the country’s best chance to save lives, reach the shared goal of herd immunity and return to a sense of normalcy.
Despite expert advisory groups, including the CDC and WHO, encouraging that as many people as possible should receive the COVID-19 vaccine, many are still hesitant to receive it. Vaccine hesitancy can have a harmful impact not only on population health, but also on healthcare budgets. Because of this, plan sponsors, whose budgets, objectives, and members’ health are of top priority, are directly impacted by vaccine hesitancy. Given that annual COVID-19 vaccinations may also be necessary looking forward, reducing vaccine hesitancy remains a priority.
What are the possible effects of vaccine hesitancy?
- A decrease in overall vaccination rates, continuing the risk of contracting or spreading infection within one’s community.
- An ongoing toll on a population’s physical and mental health, which could result in poor health outcomes or other severe complications for those affected and their families.
- An escalation of critical issues for plan sponsors such as rising healthcare costs, absenteeism, lack of productivity, revenue loss or strain on resources to cover the impact at an unsustainable pace.
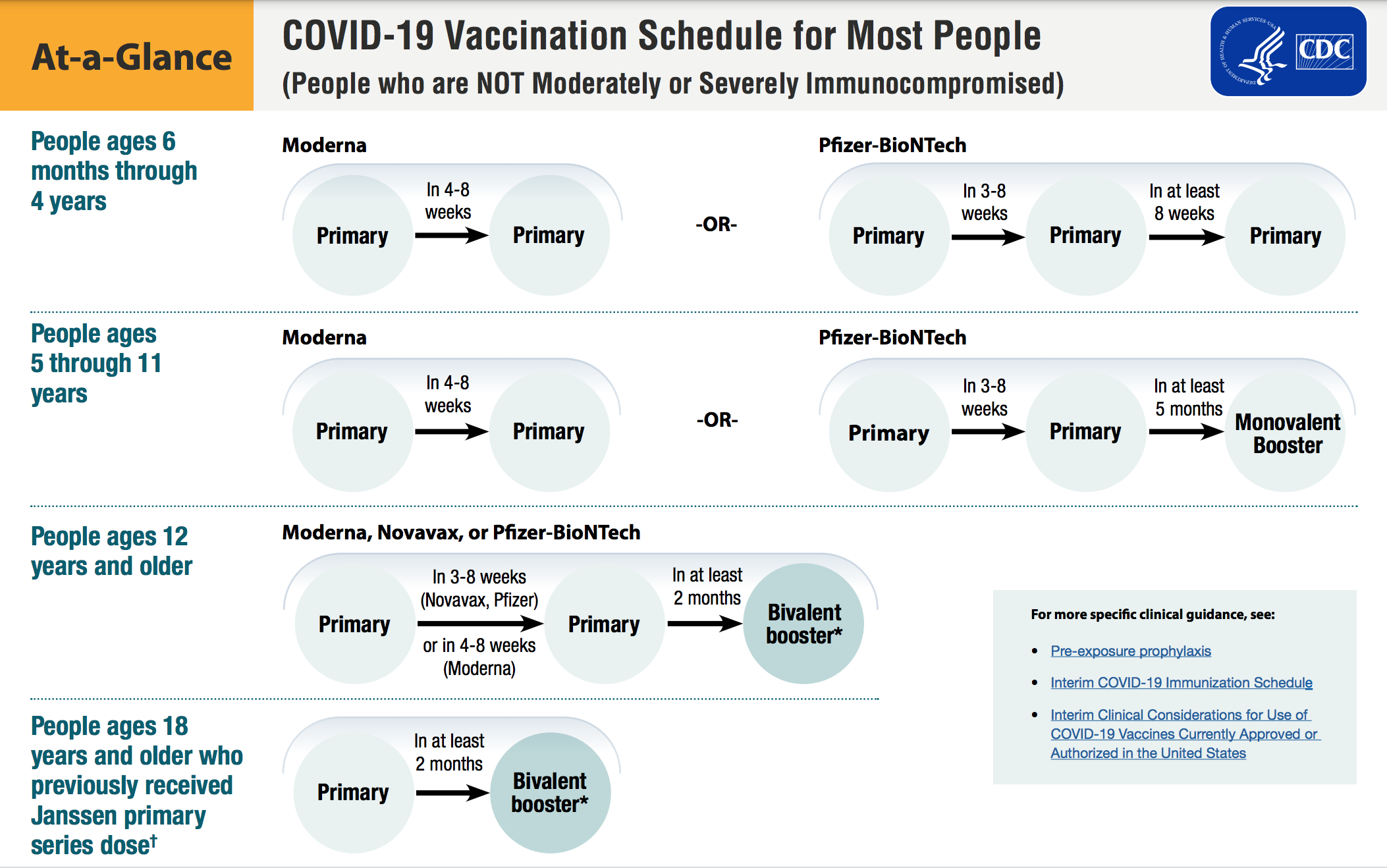
One of the most costly effects of vaccine hesitancy for plan sponsors is hospitalization. According to The Peterson-KFF Health System Tracker (2021), more than 690,000 COVID-19 hospitalizations could have been prevented by vaccination between June and November 2021. The report determined that “while real-time data on the cost of all COVID-19 hospitalizations are not publicly available, various sources point to an average cost of $20,000.” That means that in the six-month span, preventable COVID-19 hospitalizations among unvaccinated adults cost more than $13 billion in 2021. For a large plan, the possible extra costs related to poor vaccination levels could run into the millions of dollars.
So why the hesitancy?
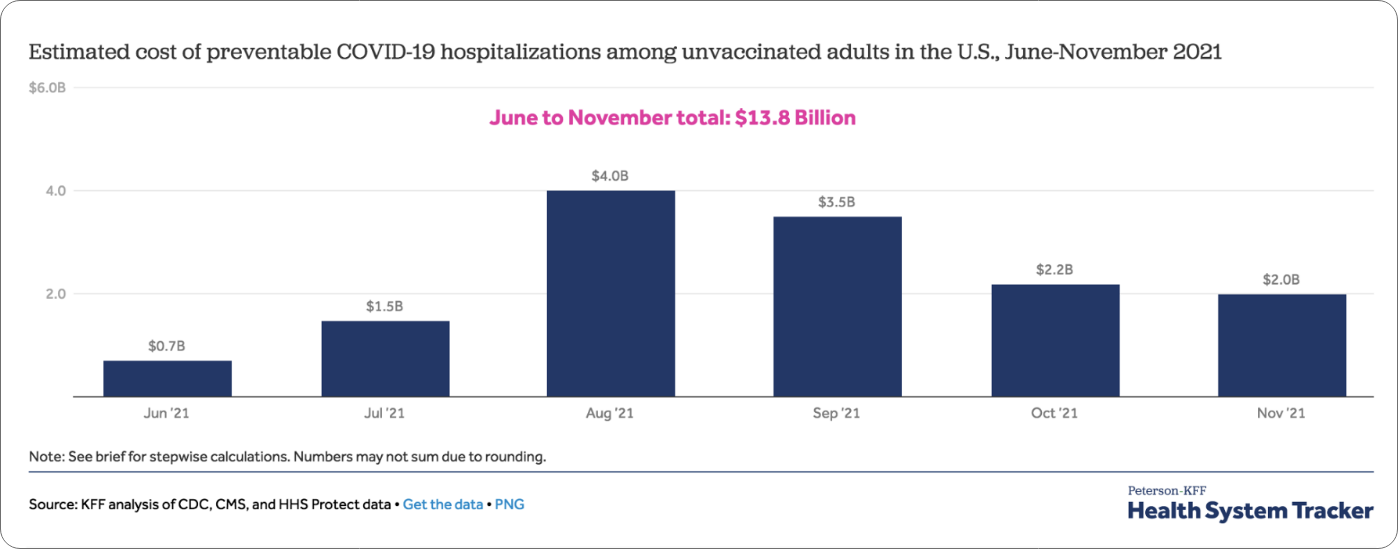
Surveys suggest many factors can impact vaccine enthusiasm: age, race and ethnicity, gender, educational background and rural vs. urban settings. The most common factors, though, are concerns, questions, and often, misinformation around COVID-19 vaccine safety, even though over 260 million people in the U.S. have received at least one COVID-19 vaccine dose (78.4% of the U.S. population) and very few severe reactions have been reported. To see the most up-to-date vaccination rates, visit the CDC COVID Data Tracker for the latest distribution information, including data in your state.
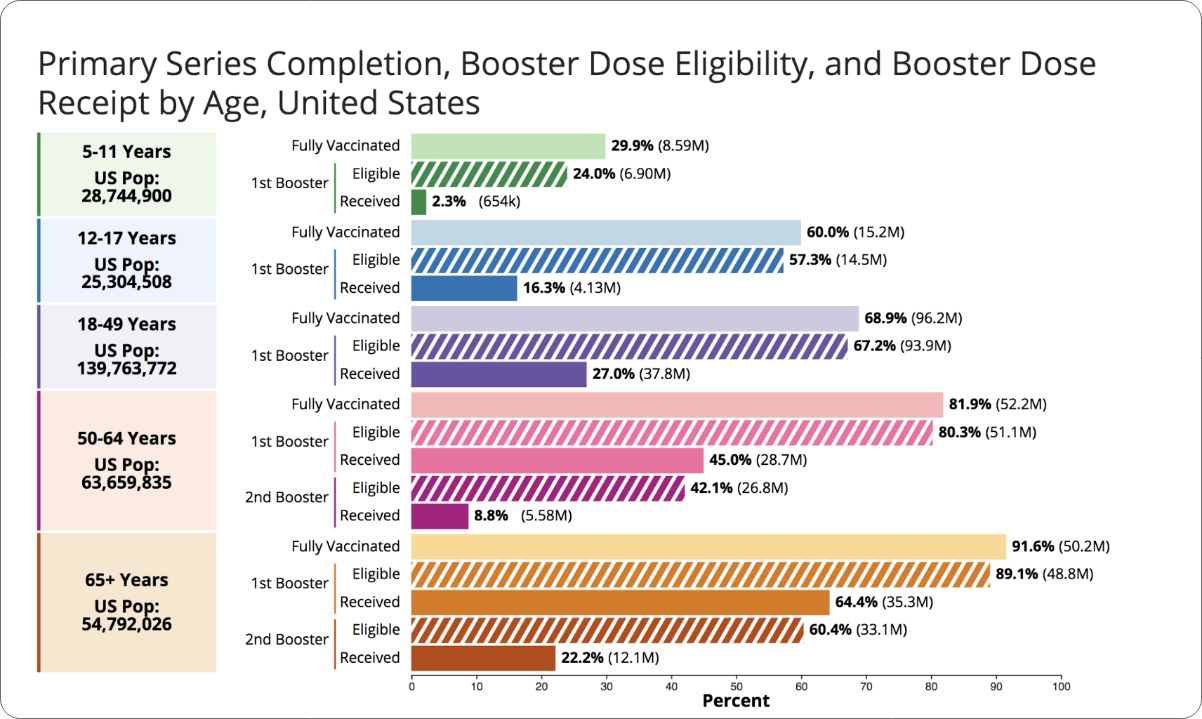
*As reported by the CDC (July 2022). “COVID-19 Vaccinations in the United States.” https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-additional-dose-totalpop
How can plan sponsors reduce vaccine hesitancy?
Plan sponsors can reduce vaccine hesitancy in their population by effectively communicating strategic messaging that encourages vaccination and alleviates safety concerns. Here are four common COVID-19 vaccine safety concerns and how to address them with clinical insight for your population.
Four common safety concerns and how to address them:
- Technology is too new While COVID-19 vaccines are new, the technology used to develop them is not. COVID-19 is the newest of many coronaviruses. For years, researchers have been studying coronaviruses and have accumulated information about this type of virus. Since the start of the pandemic, researchers quickly discovered key details about this virus to make vaccines tested in clinical trials.
- Steps were skipped in development for speed In fact, no safety steps were skipped during vaccine development. The COVID-19 vaccines were tested on tens of thousands of study participants. The careful collaboration of scientists, new technologies and government funding supported an expedited timeline for vaccine development. The U.S. Food and Drug Administration (FDA) also reviews data from clinical trials to ensure safety and efficacy before granting emergency use authorization for vaccines during this public health emergency. Safety and diligence are still top priorities as data continues to be monitored after vaccination to understand antibody development, length of protection, efficacy against virus variants and any side effects. The FDA has now granted full approval for the Pfizer-BioNTech (Comirnaty) for people aged 12+ and Moderna (Spikevax) for people aged 18+. On June 30, 2022, the FDA recommended that vaccine manufacturers add Omicron BA.4 and BA.5 spike protein components to its current vaccines to create a two-component booster. The modified vaccines could potentially be used for boosters in the U.S. starting in early to mid-fall 2022.
- Worried about side effects Mild to moderate reactions are common with the COVID-19 vaccine and usually resolve quickly. These reactions such as headache, chills, muscle aches, tiredness are similar to those experienced by some getting the flu and other vaccines and are a sign that the vaccine is working and their immune system is responding.
- Fact or fiction? Plan administrators should debunk vaccine myths by dispelling misinformation. The COVID-19 vaccine does not contain a live virus, there are no microchips in the vaccine, and COVID-19 and other vaccines will not alter a patient’s DNA.
How can plan sponsors encourage their members to get vaccinated?
Plan sponsors can use engaging ways to utilize their internal communications and channels to educate their member population.
- Show vaccine pride and tell stories that positively show the impact of their decision to get vaccinated.
- Share links to information about vaccine recommendations and local vaccine providers to promote sign-up and vaccination/boosters as soon as they are eligible.
- Share these resources or create strategic talking points to alleviate your members’ concern:
- Answers to frequently asked questions about COVID-19 vaccination
- CDC details about COVID-19 vaccination safety
- Facts to debunk and dispel myths about COVID-19 vaccination

Joshua Halperin, PharmD, Senior Clinical Director
PharmaLogic® reporting monitors COVID-19 vaccination utilization in your pharmacy benefit and showcases specific at-risk groups in your population with underlying conditions that you may need to target with a communication.
Log in to your PharmaLogic® Portal to check your COVID-19 Monitoring report for utilization of vaccinations in your pharmacy benefit and look for specific at-risk groups in your population with underlying conditions that you may need to target with a communication.
Concerned about COVID-19 vaccine hesitancy in your population?
Reach out to the Remedy team for customized data and clinical insight to help address specific factors that may be preventing your patients from getting the vaccination.