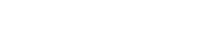PharmaLogic® Spotlight 2026: Edition 1
- | 10 min read
PharmaLogic® Spotlight communications explore evolving pharmacy dynamics and emerging trends that influence drug utilization and cost, supporting informed benefits decision-making.
Inside this PharmaLogic® Spotlight:
2025 Drug Approvals Influencing Benefits
The pace of new drug approvals picked up during the second half of 2025, bringing the total to more than 50 new drugs and biologics for the year. Many offer new therapeutic approaches to challenging diseases. Cancer remains the most represented condition for new drugs. Drugs for several rare diseases, with many being first-in-class treatments, offer new hope for those patients. Cardiovascular drug approvals include new lipid lowering options and therapies to treat arrhythmias and cardiomyopathy. A new HIV pre-exposure prevention (PrEP) drug, drugs for dry eye and presbyopia (farsightedness), an every 6-month injectable for asthma, an antibiotic to treat sexually transmitted infections, a non-opioid pain medication and the first oral GLP-1 to for weight loss also received approval from the FDA.
Review your plan use of drugs FDA approved in 2025 and YTD 2026 on the PharmaLogic® Spotlight report.
Lipid Lowering Drug Trends
Surveys suggest many factors can impact vaccine enthusiasm: age, race and ethnicity, gender, educational background and rural vs. urban settings. The most common factors, though, are concerns, questions, and often, misinformation around COVID-19 vaccine safety, even though over 260 million people in the U.S. have received at least one COVID-19 vaccine dose (78.4% of the U.S. population) and very few severe reactions have been reported. To see the most up-to-date vaccination rates, visit the CDC COVID Data Tracker for the latest distribution information, including data in your state.
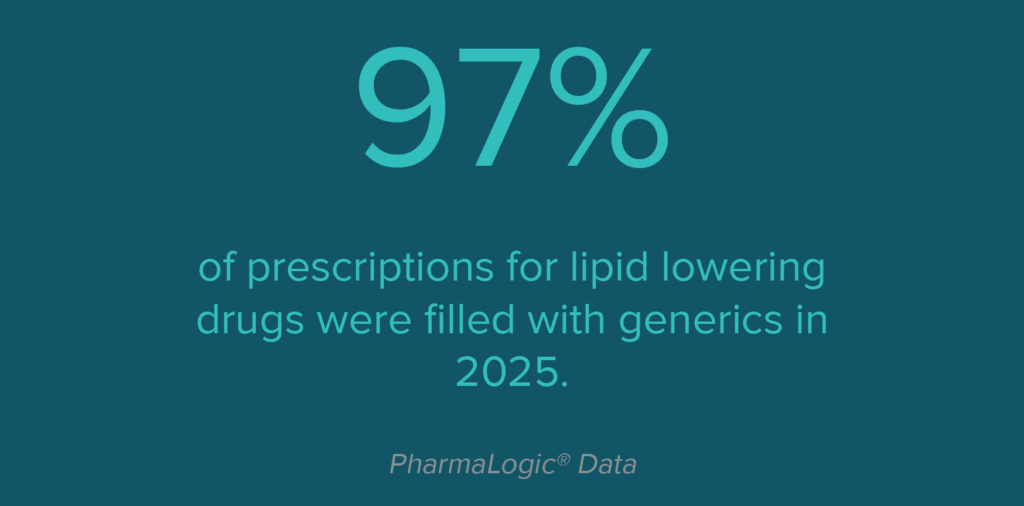
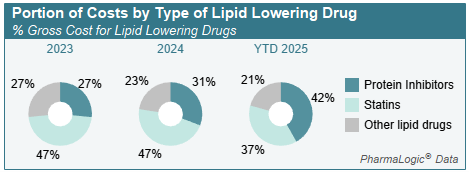
Prescribers also use other medications to block absorption of lipids and target other functions to decrease LDL (bad cholesterol), increase HDL (good cholesterol) and lower triglyceride levels. Some patients require additional medications that block or inhibit certain lipid proteins to achieve optimal cholesterol levels. These medications include Repatha®, Praluent®, Leqvio®, Redemplo® and Lerochol™ – all branded medications are under patent, with no generics currently available. Another new lipid protein blocker may receive FDA approval in 2026.
Use of lipid protein blockers has increased over the past 3 years, accounting for 42% of costs in the lipid lowering category during 2025.
Review your plan use of lipid lowering drugs via the PharmaLogic® Spotlight report.
Vaccine Guideline Revisions
Recent decisions made by the Centers for Disease Control and Prevention (CDC), the CDC’s Advisory Committee on Immunization Practices (ACIP) and the FDA signaled new directions in vaccine policy.
Updates included:
- COVID-19 vaccinations are now recommended only for people age 65 and over and patients 6 months and older with conditions that put them at high risk for severe outcomes from COVID-19. The decision to vaccinate anyone 6 months and older is now based on shared clinical decision making between the patient and provider after discussing the risks vs the benefits
- Separate MMR (measles, mumps, and rubella) and varicella (chickenpox) shots are now recommended for a child’s first dose rather than the combined MMRV shot that combined all 4 vaccines.
- Birth vaccination for hepatitis B has shifted away from a universal recommendation to a decision made by parents and their doctor. Birth vaccination does remain recommended for babies born to mothers with hepatitis B infection.
Influenza guidance has not changed. Annual influenza vaccines remain recommended for everyone 6 months and older.
Influenza and COVID-19 vaccinations rates within pharmacy benefits were lower in 2025, declining 7% for influenza and 30% for COVID-19.
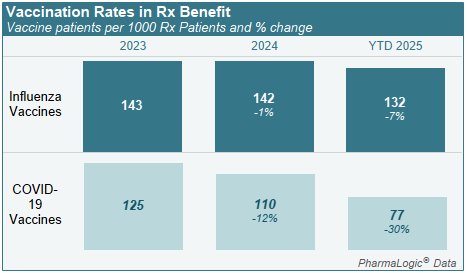
Vaccination rates in your plan are identified on the PharmaLogic® Spotlight report.
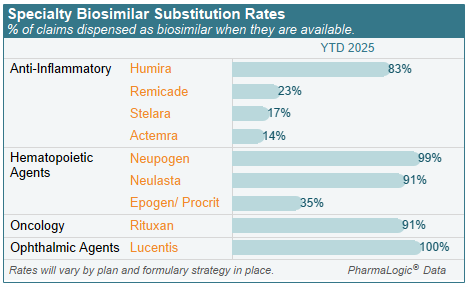
Biosimilar Use
As patents for brand biologic drugs have expired, lower cost biosimilar options have become available. Meaningful use of lower cost Humira® biosimilars was realized in most plans in late 2024 and 2025. New biosimilars for Stelara® and Prolia® became available in 2025 and use is increasing.
Formulary strategies preferring biosimilars and excluding coverage of original brands when supplies of biosimilars are stable are key to achieving savings.
Injectable Drug Growth
Injectables account for a larger portion of prescription benefit costs. Use of injectable anti-inflammatory, rare disease, respiratory, weight loss, migraine, lipid lowering and insulin drugs has grown.

Explore injectable drug use:
- Review your pharmacy plan’s use of injectables via the PharmaLogic® Injectables in Pharmacy Benefit Claims report.
- Consider discussions with medical carriers and pharmacy benefit managers to review use of injectables.
- This type of investigation may elicit opportunities for more efficient care delivery and possible savings for the overall health benefit.
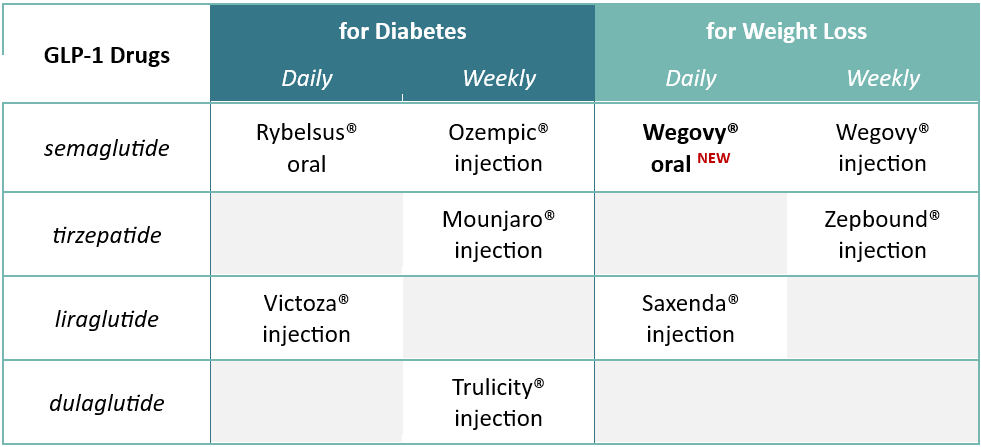
GLP-1 Update
The first oral GLP-1 for weight loss was approved in December 2025. An increase in patients using GLP-1s for weight loss is expected as the new tablet form of Wegovy enters the market. More GLP-1s, including another oral option, are pending FDA approval.
The availability of new GLP-1 drugs, supply and demand changes, competition, the entrance of many direct-to-consumer online vendors and governmental pressures have contributed to price volatility. Net cost reductions are possible for GLP-1s, but increased use will likely still continue to stress benefit plan budgets.
Remedy will assist you in discussions with PBMs and disease management providers to refine your benefit approach to weight loss and other uses for GLP-1 drugs.
Our team is ready to assist you as you face new prescription benefit challenges and contemplate benefit changes and potential solutions. Together, we can review strategies with PBMs, including optimizing formulary incentives to support biosimilar use and utilization and behavioral management related to new and continuing drug therapies.
Review your plan’s use of new drugs, biosimilars, vaccines, anti-inflammatory drugs and GLP-1s via the Spotlight and other PharmaLogic® reports.
PharmaLogic® reporting monitors COVID-19 vaccination utilization in your pharmacy benefit and showcases specific at-risk groups in your population with underlying conditions that you may need to target with a communication.
Log in to your PharmaLogic® Portal to check your COVID-19 Monitoring report for utilization of vaccinations in your pharmacy benefit and look for specific at-risk groups in your population with underlying conditions that you may need to target with a communication.